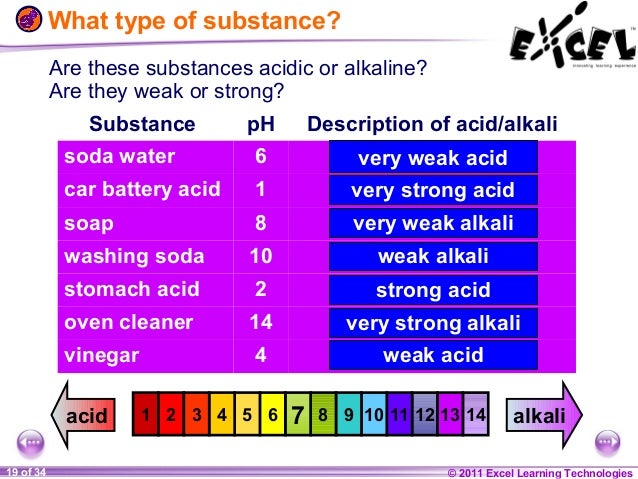
Everyday Examples of Alkaline Substances: A Comprehensive Guide
In chemistry, the pH scale is used to specify the acidity or basicity (alkalinity) of an aqueous solution. Alkaline substances, also known as bases, have a pH greater than 7.0. Understanding the prevalence and uses of alkaline substances is crucial in various fields, from household cleaning to industrial processes and even human health. This article will explore numerous examples of alkaline substances encountered in everyday life, providing a comprehensive overview of their properties and applications.
What are Alkaline Substances?
Before diving into specific examples of alkaline substances, it’s important to understand their fundamental characteristics. An alkaline substance is a chemical compound that neutralizes acids. In aqueous solutions, these substances increase the concentration of hydroxide ions (OH−). The higher the concentration of hydroxide ions, the more alkaline the substance.
The properties of alkaline substances include:
- A pH value greater than 7.
- A slippery or soapy feel.
- The ability to neutralize acids.
- Often bitter taste (though tasting chemicals is strongly discouraged).
- Reaction with acids to form salts and water.
Household Examples of Alkaline Substances
Many common household products are examples of alkaline substances. These substances are used for cleaning, cooking, and various other purposes.
Baking Soda (Sodium Bicarbonate)
Baking soda, chemically known as sodium bicarbonate (NaHCO3), is a versatile alkaline substance found in nearly every kitchen. It is commonly used in baking as a leavening agent, causing dough to rise. Beyond cooking, baking soda can be used as a household cleaner, deodorizer, and even as an antacid to relieve heartburn. [See also: Baking Soda Uses and Benefits]
Ammonia
Ammonia (NH3) is a powerful alkaline substance used in many household cleaning products. It is particularly effective at removing grease and grime from surfaces like glass and tile. However, ammonia should be used with caution, as it can be irritating to the skin and respiratory system. It should never be mixed with bleach, as this can create toxic fumes.
Bleach (Sodium Hypochlorite)
Bleach, typically sodium hypochlorite (NaClO), is another common alkaline substance used for disinfecting and whitening. It is used to clean surfaces, remove stains from laundry, and kill bacteria. Like ammonia, bleach should be handled with care and never mixed with other cleaning products.
Soaps and Detergents
Most soaps and detergents are alkaline substances. They work by emulsifying fats and oils, allowing them to be washed away with water. The alkalinity of soaps helps to break down grease and dirt, making them effective cleaning agents. Different types of soaps and detergents have varying levels of alkalinity, with some being milder than others.
Drain Cleaners
Drain cleaners often contain strong alkaline substances like sodium hydroxide (NaOH), also known as lye. These substances work by dissolving organic matter that clogs drains, such as hair and grease. Drain cleaners are highly corrosive and should be used with extreme caution, following all safety instructions.
Industrial Examples of Alkaline Substances
Alkaline substances play a vital role in various industrial processes. Their properties make them essential for manufacturing, water treatment, and chemical production.
Sodium Hydroxide (Lye or Caustic Soda)
Sodium hydroxide (NaOH) is a strong alkaline substance widely used in industry. It is used in the production of paper, textiles, soaps, and detergents. It is also used in the refining of petroleum and the processing of aluminum. Sodium hydroxide is a highly corrosive substance and must be handled with appropriate safety measures.
Potassium Hydroxide (Caustic Potash)
Potassium hydroxide (KOH) is another strong alkaline substance used in various industrial applications. It is used in the production of soft soaps, liquid fertilizers, and alkaline batteries. It is also used as an electrolyte in alkaline batteries and in the food industry as a pH regulator. [See also: Potassium Hydroxide Applications]
Calcium Hydroxide (Slaked Lime)
Calcium hydroxide (Ca(OH)2), also known as slaked lime or hydrated lime, is an alkaline substance used in construction, agriculture, and water treatment. In construction, it is used in mortar and plaster. In agriculture, it is used to neutralize acidic soils. In water treatment, it is used to raise the pH of water and remove impurities.
Sodium Carbonate (Soda Ash)
Sodium carbonate (Na2CO3), also known as soda ash, is an alkaline substance used in the manufacture of glass, detergents, and paper. It is also used as a water softener and as a pH regulator in various industrial processes.
Alkaline Substances in Nature
While many alkaline substances are manufactured, some occur naturally. These substances play important roles in environmental processes and geological formations.
Limestone (Calcium Carbonate)
Limestone is a sedimentary rock composed primarily of calcium carbonate (CaCO3). While calcium carbonate itself is not highly alkaline, it can react with water to form calcium hydroxide, which is an alkaline substance. Limestone is used in construction, agriculture, and the production of cement.
Certain Soil Types
Some soils naturally have a high pH due to the presence of alkaline substances such as calcium carbonate and magnesium carbonate. These soils are often found in arid and semi-arid regions. The alkalinity of the soil can affect plant growth, as some plants thrive in alkaline conditions while others prefer acidic conditions.
Seawater
Seawater is slightly alkaline, with a typical pH range of 7.5 to 8.4. The alkalinity of seawater is due to the presence of various dissolved salts, including carbonates and bicarbonates. This alkalinity helps to buffer the ocean against changes in pH, protecting marine life from the effects of acidification.
Alkaline Substances and Human Health
The concept of alkalinity extends beyond chemistry and into the realm of human health. While the idea of an “alkaline diet” is often debated, understanding the role of alkaline substances in the body is important.
Antacids
Antacids are medications used to neutralize stomach acid and relieve heartburn. They typically contain alkaline substances such as calcium carbonate, magnesium hydroxide, or aluminum hydroxide. These substances react with stomach acid to reduce its acidity, providing relief from symptoms of acid reflux.
Blood pH
The human body maintains a tightly controlled blood pH, which is slightly alkaline (around 7.4). Various physiological mechanisms work to maintain this pH balance, as even small deviations can have serious health consequences. The kidneys and lungs play a crucial role in regulating blood pH by controlling the levels of bicarbonate and carbon dioxide in the body.
The Alkaline Diet
The alkaline diet is a dietary approach based on the idea that consuming alkaline substances can help to balance the body’s pH and improve health. Proponents of the alkaline diet suggest eating more fruits, vegetables, and certain grains, while limiting acidic foods such as meat, dairy, and processed foods. While some studies suggest potential benefits, the scientific evidence supporting the alkaline diet is limited, and more research is needed.
Safety Considerations When Working with Alkaline Substances
Many alkaline substances can be corrosive and harmful if not handled properly. It is essential to follow safety precautions when working with these substances to prevent injury.
- Always wear appropriate protective gear, such as gloves, goggles, and a lab coat.
- Work in a well-ventilated area to avoid inhaling fumes.
- Never mix alkaline substances with acids or other chemicals unless specifically instructed to do so.
- Store alkaline substances in clearly labeled containers and keep them out of reach of children.
- In case of contact with skin or eyes, rinse immediately with plenty of water and seek medical attention.
Conclusion
Alkaline substances are prevalent in everyday life, from household cleaning products to industrial processes and even in the natural world. Understanding their properties and applications is essential for various fields, including chemistry, industry, and human health. By recognizing the examples of alkaline substances around us, we can better appreciate their importance and use them safely and effectively. Whether it’s baking soda in the kitchen, sodium hydroxide in industrial manufacturing, or the alkalinity of seawater, these substances play a vital role in shaping our world. This comprehensive guide provides a solid foundation for further exploration and understanding of the fascinating world of alkaline chemistry.
